"Buy cheapest zithromax, antibiotics for recurrent uti in pregnancy".
By: I. Basir, M.S., Ph.D.
Associate Professor, Midwestern University Arizona College of Osteopathic Medicine
Text books and biochemical research literature indicate its uses in biochemical techniques and chemical literature reviews its synthesis and uses in chemical synthesis bacteria killing products discount zithromax 500 mg without prescription. By appearance it is a clear xelent antibiotic order zithromax 100 mg fast delivery, colorless infection 7 weeks after surgery discount 250 mg zithromax otc, crystalline (but soft bacterial diseases purchase 500 mg zithromax with visa, waxy) solid that is volatile with pungent, acidic and irritating vapor. It has a low melting point of 51 °C and vapor pressure is one atmosphere at 61 °C. It is slowly soluble in water and very soluble in acetonitrile, a 50% solution is often used, and can Laboratory Safety Guide 368 Miscellaneous Anecdotal Information be purchased as such. It is made by bromine oxidation of cyanide salt in aqueous solution, whether dilute or concentrated, and can be distilled into a receiver as a pure compound or kept as an aqueous solution if to be used soon. Triple bonds of small atoms are generally reactive towards polymerization or cyclic trimerization and this molecule is endothermic, its elements are more stable than the compound is in the gas phase. The reaction may be an oligomerization or polymerization, possibly caused by a trace of cyanide salt in the crystaline solid. If so, the cyanide, being basic (nucleophillic) starts the reaction by bonding its "C" end to the carbon of cyanogen bromide and the charge now shifted to the "N" end will bond to another carbon, and so it repeats. There is much extra energy to be had, particularly in energy per heat capacity that translates to a great rise in temperature. A slower decomposition hazard that can result in eventual bursting is when the container closure allows atmospheric humidity to enter. Hydrolysis of cyanogen bromide then occurs, first to isocyanic acid, and then with trimerization to cyanuric acid, a white, opaque, water insoluble solid, and hydrogen bromide, which will exist as a pressurizing gas (it is not trapped by anything basic, condensed or released, unless the cap is too lose) that can make the container a danger on being opened. Viewing the material through the glass and observing the contents to be opaque, as opposed to being clear, will have to do as a warning that hydrolysis has occurred here. Methylthiocyanate is released when the carbonyl oxygen of methionine displaces it, forming an iminolactone five member ring with the four carbon atoms of methionine, which then hydrolyses to cleave the peptide. Cyanide is not observed in these cases to be released as such, it takes the nucleophille, bromide takes the electrophille. In the case of water hydrolysis, hastened by addition of hydroxide, the observation will be consistent with the observed reactions in its use as a reagent; cyanide takes the nucleophille and bromide is freed to solution as such. Oxygen, if formed could throw the equilibrium by just leaving the scene, an end reaction. Its easy to demonstrate that cyanide is not a product; one gram of cyanogen bromide (in good condition) added to about 30 milliliters of water (takes a long time to dissolve) will give strong odor of same as it is pushed to dissolve. Add 2 N base as though titrating and observe that violet basic color will slowly give way to neutral color as more cyanogen bromide dissolves. Repeat the portionwise addition until the crystal is gone and violet color persists. Give the solution some time and notice that cyanogen bromide odor is nearly vanished (be careful in any event). If cyanide were the result of hydrolysis, the test, however done would be strongly indicating. There is more information available on the toxicity of cyanogen chloride than there is for the bromide. Cyanogen bromide was developed to be a safer and easier to use form of the chemical in chemical synthesis and some biochemical applications. Being in high volume production and having a lethal history, the chemical was studied. Research in the late forties and early fifties demonstrated that cyanogen chloride reacts as expected with a mercaptan function in glutathione, forming a thiocyanate and hydrogen chloride as products. A second glutathione mercaptan then displaces the cyanide of the thiocyanate, forming a disulfide between two glutathiones and hydrogen cyanide is released. Cyanide blocks the electron transport chain of proteins and cofactors at the end of the line; just before electrons are donated to respired oxygen, rendering it unusable and stopping the electron current. The toxicity of cyanogen chloride, bromide and iodide is thus the same as cyanide. There is a difference in that hydrogen cyanide is more lethal in high concentrations for short times than it is with lower concentrations and longer times, where concentration times time is equal in both cases. Cyanogen chloride or bromide, because of its mechanism being slower, will build its dose over time, in lower concentrations than cyanide can act. Some of the components must be taken intravenously, which is tricky, especially when time is tight. The usual strategy is to get hemoglobin to be a superior sequesterer of cyanide by oxidizing its iron to +3, methemglobin, using a nitrite ester inhalation or sodium nitrite injection.
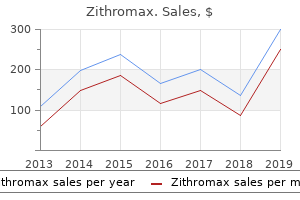
The output of styrene resins in 1961 was 1 infection 13 lyrics purchase discount zithromax on line,145 million pounds; sales totaled 1 virus hitting schools order zithromax from india,079 million pounds antibiotics for uti erythromycin discount zithromax 500mg on line, valued at $282 million virus free screensavers order 500mg zithromax overnight delivery. S, production and sales, by chemical composition, 1961 Plaantities and values are given in terms of the total weight of the materials (dry basis). Listed below are all plastics and resin materials for which any reported data on production or sales may be published. For the purposes of this report, "dry basis" is defined as the total weight of the material, including resin, plasticizers, fillers, extenders, colors, and stabilizers, and excluding water, solvents, and other liquid diluents. Styrene-alkyd polyesters for protective coatings are included under "Styrene resins. Production of these resins in 1961 was 665 million pounds; sales amounted to 544 million pounds, valued at $149 million. The phthalic alkyd resins, used principally in the manufacture of protective coatings, were third in volume of production in the benzenoid group; production in 1961 amounted to 447 million pounds. The output of epoxy resins in 1961 was 70 million pounds; that of polyester resins was 193 million pounds. Production of nonbenzenoid plastics and resins in 1961 amounted to 3,881 million pounds, compared with the 3,427 million pounds reported for 1960. Sales of these resins in 1961 amounted to 3,640 million pounds, valued at $1,077 million, compared with 3,119 million pounds valued at $1, 025 million, in 1960. The output of polyethylene resins amounted to 1,606 million pounds in 1961, compared with 1,337 million pounds in 1960. Sales of polyethylene resins in 1961 totaled 1,582 million pounds, valued at $388 million, compared with 1,195 million pounds, valued at $343 million, in 1960. In this report, statistics are given for production and sales of polyethylene resins produced by both the high-pressure and the low-pressure processes. The output of vinyl resins in 1961, which ranked next to that of polyethylene resins, amounted to 1,260 million pounds, compared with 1,203 million pounds in 1960. Sales of vinyl resins in 1961 totaled 1,212 million pounds, valued at $307 million, compared with 1,130 million pounds, valued at $329 million in 1960. While the group totals are in substantial agreement with those given in table 15A, the data are partially estimated, and may not be correlated exactly with those given in that table. Changes in classification and an increase in coverage on some products may result in differences between the detail figures given in the above table and those given in the January 1962 release. Other important resins in the nonbenzenoid group are the acrylic, polyamide, polypropylene, silicone, and nonphthalic alkyd resins. The largest single use reported for plastics materials in 1961, as in previous years, was for the molding and extrusion of finished and semi finished articles. Other important uses for which statistics are shown are for adhesives, treat· ment of textiles and paper, protective coatings, and bonding materials. Production of cellulose plastics as a group amounted to 148 million pounds in 1961. Rubber Processing Chemicals - Rubber-processing chemicals are organic compounds that are added to natural and synthetic rubbers to give them qualities necessary for their conversion into finished rubber goods. Statistics on production and sales of rubber-processing chemicals in 1961 are given in table 17A. The larger total output of rubber-processing chemicals in 1961 is attributable principally to increased production of amino antioxidants. Sales of rubber-processing chemicals in 1961 amounted to 156 million pounds, valued at $104 million, compared with 153 million pounds, valued at $101 million, in 1960. The output of cyclic rubber-processing chemicals in 1961 amounted to 174 million pounds, or 1. Sales in 1961 were 135 million pounds, valued at $89 million, compared with 130 million pounds, valued at $85 million, in 1960. Of the total output of cyclic rubber-processing chemicals in 1961, accelerators accounted for 38. Accelerators, principally dithiocarbamic acid derivatives and tetramethylthiuram sulfides, accounted for about 55. Peptizers and modifiers-chiefly dodecyl mercaptans-together with blowing agents and lubricating and conditioning agents, accounted for 44. Includes small quantities produced and sold for uses other than rubber processing. Data on production and sales of aldehyde and acetone amine antioxidants are included below in "All other cyclic rubber-processing chemicals.

Meira Ziv is a Professor in the Robert H Smith Institute of Plant Science and Genetics at the Hebrew University of Jerusalem (Israel) antibiotics human bite order zithromax with american express. Her research interests are in the physiology and morphogenesis of plant organogenesis and somatic embryogenesis in large scale liquid cultures; shoot-malformation antibiotic bactrim uses 250mg zithromax overnight delivery, hyperhydricity and the role of oxidative stress in the control of plant development in bioreactor cultures for efficient acclimatization and survival ex vitro; bulb and corm development in geophytes cultured in liquid cultures in relation to carbohydrate metabolism 3m antimicrobial oral rinse generic zithromax 100 mg without a prescription. Jianxin Chen is a research scientist in the Department of Biology at Brock University antibiotic resistance world map best zithromax 500mg, Ontario (Canada). His interests are in large-scale micropropagation, metabolic pathways and cloning of medicinal plants and plant breeding. It includes techniques and methods used to research into many botanical disciplines and has several practical objectives. This chapter therefore describes the techniques that have been developed for the isolation and in vitro culture of plant material, and shows where further information can be obtained. It occurs when plant organs such as the growing points of shoots or roots (apical meristems), leaf initials, young flower buds or small fruits, are transferred to culture and continue to grow with their structure preserved. This may occur in vitro either directly upon an organ or upon a piece of tissue placed in culture (an explant), or during the culture of previously unorganised tissues. The process of de novo organ formation is called the growth of higher plants depends on the organised allocation of functions to organs which in consequence become differentiated, that is to say, modified and specialised to enable them undertake their essential roles. Unorganised growth is seldom found in nature, but occurs fairly frequently when pieces of whole plants are cultured in vitro. The cell aggregates, which are then formed, typically lack any recognisable structure and contain only a limited number of the many kinds of specialised and differentiated cells found in an intact plant. A differentiated cell is one that has developed a specialised form (morphology) and/or function (physiology). So far, the formation of differentiated cell types can only be controlled to a limited extent in culture. It is not possible, for example, to maintain and multiply a culture composed entirely of epidermal cells. By contrast, unorganised tissues can be increased in volume by subculture and can be maintained on semisolid or liquid media for long periods. Differentiation is also used botanically to describe the formation of distinct organs through morphogenesis. It includes the aseptic isolation from whole plants of such definite structures as leaf primordia, immature flowers and fruits, and their growth in vitro. For the purposes of plant propagation, the most important kinds of organ culture are: · Meristem cultures, in which are grown very small excised shoot apices, each consisting of the apical meristematic dome with or without one or two leaf primordia. These shoot apices are usually cultured in such a way that each produces multiple shoots. The growth of roots, unconnected to shoots: a branched root system may be obtained. In practice the following kinds of cultures are most generally recognised: · Callus (or tissue) cultures. The growth and maintenance of largely unorganised cell masses, which arise from the uncoordinated and disorganised growth of small plant organs, pieces of plant tissue, or previously cultured cells. Populations of plant cells and small cell clumps, dispersed in an agitated, that is aerated, liquid medium. The objective is usually to obtain haploid plants by the formation of somatic embryos (see below) directly from the pollen, or sometimes by organogenesis via callus. Pollen cultures are those initiated from pollen that has been removed from anthers. The derivation of new plants from cells, which would not normally have taken part in the process of regeneration, shows that living, differentiated plant cells may express totipotency, i. Nevertheless, as will be described in the chapters, which follow, a very large measure of success can be achieved and cultures of various kinds can be used to propagate plants. Explants the objective of plant propagation via tissue culture, termed micropropagation, is to propagate plants true-to-type, that is, as clones.
Aqueous solutions of inorganic chemicals should be disposed of according to Sanitary Sewer 6 antibiotic yeast infection symptoms buy zithromax us. Aqueous solutions of organic chemicals should be disposed according to Sanitary Sewer 7 antibiotics vs antibacterial zithromax 500 mg generic. Bases Look up the chemical in Appendix A virus 72 hours buy 500 mg zithromax otc, then see Neutralization Procedures: Strong Acids and Bases antibiotics rash buy zithromax 100 mg line. Batteries There are four types of batteries used on campus: automotive, ordinary alkaline, toxic metal containing. Each of these battery types requires a different disposal procedure: Batteries containing toxic metals like mercury and cadmium. Lithium metal nickel-metal hydride containing batteries may be hazardous (flammable) and must be disposed following procedure On-Site Service I. Disposal of automotive (lead-acid) and smaller lead-acid batteries involves contacting the University Garage for specific instructions. Chemical Carcinogens and Mutagens Chemical carcinogens or mutagens may be disposed of by following procedure OnSite Service I for removal by Safety Department personnel. Alternatively, you may treat certain carcinogens and mutagens, depending on the identity and quantity of waste. Laundry bleach, aqueous sodium hypochlorite-sodium chloride-sodium hydroxide mixture, is often seen as a panacea treatment of toxics and smellies. Questions to answer before bleaching your substance are: Does the chemical have the right functional group? Another important question to answer is: Is the chemical water soluble and is it compatible with aqueous base. Toxic compounds in aqueous or organic solvent may have added hazard due to the mobility of the solvent, especially in the event of a breakage or spill. Being able to chemically eliminate the toxicity and sewer dispose the water or commingle the solvent. Laboratory Safety Guide 154 Chemical Disposal Procedures see Organic Solvent Collection) could be an advantage. Specific procedures for degradation or destruction of other carcinogens and mutagens can be obtained by calling a chemist at Safety. Materials for which there are no treatment methods can be disposed following procedure On-Site Service 1 in Section 7. Carbon dioxide in air will dissolve in cyanide solutions and slowly acidify cyanide ion to hydrogen cyanide, which is volatile and is the odor of such solutions. Large volumes of solutions, high concentrations and solid salts are best disposed of following procedure On-Site Service 1. For smaller quantities of lesser concentration the following procedure may be used. For each for small volumes of dilute solutions chemical or solute, limit daily discharges to 100 grams per principal investigator. Follow procedures described in Planning For Neutralization of Acids and Bases found in Neutralization Procedures, below. Dilute the solution with water to a concentration not to exceed 2% w/v of cyanide ion. For each 50 mL of this cyanide solution slowly add 70 mL household bleach while stirring. If cyanide remains add more bleach to the reaction mixture and repeat the test for residual cyanide. If no precipitate is formed wash the solution down the sanitary sewer with 20 volumes water per volume reaction mixture. University of Wisconsin-Madison Safety Department (608) 262-8769 Chemical Disposal Procedures 155 Bleach can lose its chlorine if carbon dioxide from the air gets in the container during storage. Ethidium Bromide 1: Aqueous solutions of ethidium bromide Aqueous run-off from gel staining can be sewered without concern. Dilute aqueous solutions less than 10 mg/L can be disposed of in the sanitary sewer. Alternatively, you can use bleach to chemically treat ethidium bromide and dispose of the resulting mixture in the sanitary sewer. Add 4 mL fresh bleach for every 1 mg ethidium bromide (remember, bleach can deteriorate upon exposure to air).

In nature antimicrobial pens discount 500 mg zithromax visa, it has been found in northeastern Japan antibiotic 7 day order zithromax 100 mg fast delivery, the United States antibiotics for sinus infection not working buy cheapest zithromax and zithromax, and in the temperate regions of China and Europe negative effects of antibiotics for acne zithromax 500mg with mastercard. Temperature: the range of temperatures for mycelial growth is relatively wide from 5 to 32C and optimum temperature is 20 to 25C. The temperatures for fruiting body development range from 10 to 25C and the optimum is 18 to 22C. It is the same as with other cultivated edible mushrooms in that fruiting bodies develop slowly at lower temperatures and grow faster at higher temperature. Humidity: the water content in the substrate should be maintained in the range of 60 to 63%. During the mycelial running stage, the relative humidity of the air in the culture room should not be too high to avoid development of mold. However, during the stage of fruiting body development, the relative humidity in the culture room should be higher, that is, maintained at 85% or above. The optimum is 85 to 95% and when lower than 80%, the fruiting bodies dry out easily, particularly at the pinhead stage. Light: During fructification good illumination of light in the culture room is absolutely necessary for development of the normal size and color of the mushrooms. Even though the fruiting bodies are formed, they often become abnormal in shape and color, light or white. Air: Grifola frondosa is like Agaricus blazei and is an aerobic (aerophilic) mushroom similar to A. In the culture room, an exchange of air should be made at least five to six times every day. However, the former is a mushroom of the tropics and subtropics and requires warmer temperature and higher humidity than the latter, which is a common edible mushroom of temperate climate. In Brazil, sugar cane bagasse solely, or mixed with rice straw, was found to be the best culture bed material and the cultivation of this mushroom is carried out mainly outdoors. Although this mushroom was first introduced to Japan from Brazil in 1965, the mushroom in dry form and the raw materials for powders or tablets are now mainly imported to Japan either from Brazil or from China. This novel mushroom for both edible and medicinal uses was introduced from Japan to China in 1992. The Plant Protection Research Institute of the Agriculture Academia Sinica at Fujian Province conducted the experimental trials first. After 2 years of small-scale trials, the method with compost made of bagasse or rice straw or a mixture of wheat or rice bran, soybean powder, superphosphate of lime, and added water was found in these trials to be practicable. In 1994, the cultivation of this mushroom was extended to other areas of Fujian Province. Now it is cultivated in many parts of China during the warm summer months on relatively small-scale farms. In recent years, this mushroom has also been cultivated in Korea, Thailand, Indonesia, and the United States. Cultivation was first started in Japan, and then later the techniques spread to China and Korea. Now cultivation has gradually expanded to other parts of the world including the United States. Three cultivation methods, bottle culture, bag culture, and outdoor bed culture, have been described in detail by Mayuzumi and Mizuno. Bottle cultivation: this method is suitable for year-round cultivation and can be mechanized with minimum labor requirements. The mushrooms are usually small because of the limited nutrients available in the bottles. Hardwood sawdust is mixed with rice and wheat bran as basal ingredients of the culture substrate, which is packed in a polypropylene bag that is molded into a square shape. Outdoor bed cultivation: this method was first attempted in Japan under natural climatic conditions. This method requires more time (about 6 months) from spawning to fruiting body formation. Details are given for the process of cultivation, including selection of strains, types of spawn, where to obtain the spawn, where to obtain bags for mushroom cultivation, and substrate formulation. O2) for each growth stage are constructed in tabular form, readily accessible for application. If the cultivation bag or bed is indoors, close attention should be given to regulate temperature, relative humidity, light, and air, with air exchange adjusted by ventilation.
Generic zithromax 250mg without a prescription. Lab Dip Visual Color Assessment.